Ocean Acidification – Tiny Number has BIG Effect: 0.1 = 30%
If you have not yet heard the phrase “Ocean Acidification” it is likely that you will soon. It is a relatively new phenomenon that now has marine scientists VERY concerned. Over the next century many climate and ocean scientists believe it could be the single most important change on our planet. It’s worth an explanation.
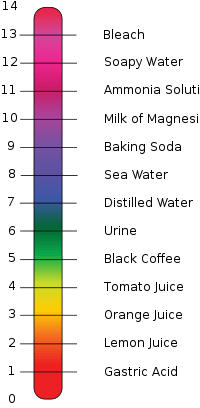
As you might recall from school chemistry, pH is a scale, essentially from 0 – 14 indicating the acidity or alkalinity level of liquids. This scale may help to visualize pH, with some examples. Anything above 7 is alkaline; anything below is acidic. Fresh water is neither acid nor alkaline, it is neutral with a pH of 7. Sea Water is shown as having a pH of roughly 8.
More accurately the water near the ocean surface is about 8.2 on the pH scale.Even most scientists believed it to be a rather static number until an article was published in SCIENCE Magazine in 2004 (Feely et al). Until then, pH was kind of boring.
That put a spotlight on what a few specialists had started to notice — over about two centuries, coinciding with the industrial revolution, and burning fossil fuels, ocean pH had changed, decreasing by about 0.1 units. While still alkaline, it was moving in the direction of the acid side of the scale. So “acidification” is a little misleading since it will likely be alkaline for centuries, just less so.
What’s the problem then? A few things — BIG things in fact.
First, we need to try to understand that pH is what’s called a logarithmic scale. As you likely know, the other phenomenon that we hear about that is rated on a logarithmic scale are earthquakes. “Log scales” are used when the actual units being measured are BIG numbers that change exponentially.
A change of one full “log” number is a TEN TIMES increase in the amount. For example, according to the scale at the left, lemon juice is about one number less than orange juice, which would translate to lemon juice having ten times the acidity as orange juice. (Knowing the difference in our mouth, what we might call “pucker power”, that relative amount of acidity likely even sounds reasonable.)
A change of one tenth of a unit of pH — 0.1 — means about a 30% change. In other words the ocean acidity has increased a very substantial 30%, rapidly accelerating over the last two centuries.
If you are a gardener, you likely know that the pH of soils are very important for what will grow. For example if you want to grow a type of rose bush, the pH has to be just right. Swimming pools are routinely tested for Chlorine level and pH. Keeping the pH in the right range makes the chlorine more effective and keeps the algae from growing.
But in the ocean we NEED THE ALGAE TO GROW. That plant matter is literally the base of the entire ocean food chain. Phytoplankton, tiny little bits of algae, may seem insignificant, but consider that they are the food of planktonic animals, which in turn are eaten by lots of marine life (including the biggest animal on our planet, the blue whale.) By the way, of possible interest to the humans reading this, phytoplankton also produce about half of the oxygen that you need to breathe. Is there a lot of it? Yes, but it is showing some marked decreases in recent decades and pH is indicated as one reason for the decline.
Increasing acidity means a few other problems.
Structures made of calcium carbonate (and related forms) include coral reefs, shellfish, as well as many of the tiny building blocks and filtering structures in the sea.
Calcium carbonate structures depend on alkaline water. They simply will not form in acid water. That effect is already being seen in some places. For example, the extremely tiny beginnings of oysters, clams and other shellfish are very delicate and it turns out quite sensitive to pH.
I just returned from Seattle and a workshop put on by E2 – Environmental Entrepreneurs. The focus of the event was to explain how oyster and clam farming (big industries, in the Pacific NW, producing more than $100 million annually) are being affected by acidification. During the last few years the oyster farmers were having a very difficult time getting their seedlings to grow — actually called “spat” at that stage. They finally identified low pH as a cause. They were getting VERY low pH, in some cases down in the 7.6 range and lower, due to upwellings of water from deep Pacific currents. (With assistance from Senator Maria Cantwell, the Sustainable Fisheries Partnership, NOAA and the Marine Conservation Institute, they now monitor the timing of those unusually low levels and avoid pumping that water into their farming tanks. But that is only a short term solution.)
Lower pH is a direct result of more carbon dioxide (CO2) disolving in the ocean. As you know CO2 levels are increasing — presently 394 and climbing; CO2 is one of the dominant greenhouse gases. See one of my earlier blogs “Best Image to Illustrate Climate Problem” for an explanation and simple graph showing our skyrocketing CO2 levels.
As CO2 increases in the atmosphere, it disolves in the ocean – simple chemistry and physics. Actually you all have tasted it. Any soda water, particularly the unflavored variety such as Perrier, tastes a little bitter or acid. That’s due to the natural carbonation — disolved carbon dioxide — that makes any “sparkling water.” When we take the cap off, we reduce the pressure of CO2 on top of the liquid. Less is able to stay in solution and turns into bubbles, rising to the top.
The bottom line is that changing pH is a big change. It will affect the entire ocean ecosystem over the course of decades and centuries. It has happened before but over very long timescales. Our current level of pH has not occurred for about 650,000 years. The best estimates are that on our current path that we could have a drop of 0.4 units during this century. That would be a level not seen for about 20 million years and would have a disastrous impact on the marine ecosystem.
What is really different now is the rate of change. The rate of change now is on the order of a hundred times faster than the previous drop 650,000 years ago, and even faster than the most recent mass extinction event 55 million years ago (Guinotte and Fabry, 2008). Back then, it was a ghastly soup totally unlike the oceans of that we have come to know. Most species went extinct.
As they say, “we don’t want to go there.” It all comes down to that level of CO2 in the atmosphere. We need to deal with it sooner, rather than later. If we continue to burn all the coal and other fossil fuels we could reach CO2 levels above 700 ppm later this century, a truly catastrophic level.
We need to find ways to avoid that at all costs.

